Electrolysis of water produces hydrogen and oxygen.
Primary tabs
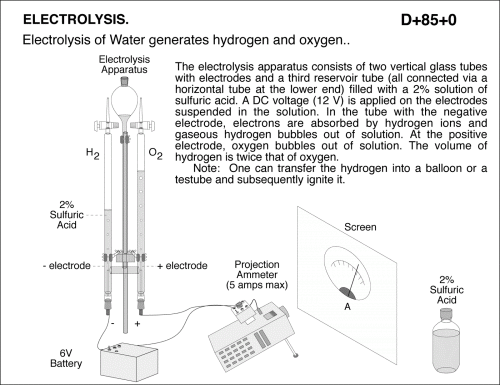
Electrolysis of Water generates hydrogen and oxygen. The electrolysis apparatus consists of two vertical glass tubes with electrodes and a third reservoir tube (all connected via a horizontal tube at the lower end) filled with a 2% solution of sulfuric acid. A DC voltage (12 V) is applied on the electrodes suspended in the solution. In the tube with the negative electrode, electrons are absorbed by hydrogen ions and gaseous hydrogen bubbles out of solution. At the positive electrode, oxygen bubbles out of solution. The volume of hydrogen is twice that of oxygen. Note: One can transfer the hydrogen into a balloon or a testube and subsequently ignite it.
UCB Index:
D+85+0
PIRA Index:
5E20.10
UCB Taxonomy:
PIRA Taxonomy:
Popularity:
- Log in to post comments