Storage cell: Gotham cell is charged up and rings a bell.
Primary tabs
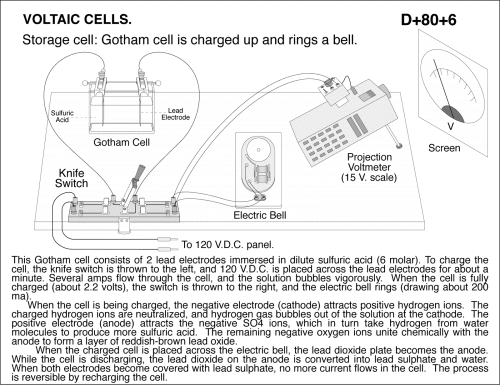
Storage cell: Gotham cell is charged up and rings a bell. This Gotham cell consists of 2 lead electrodes immersed in dilute sulfuric acid (6 molar). To charge the cell, the knife switch is thrown to the left, and 120 V.D.C. is placed across the lead electrodes for about a minute. Several amps flow through the cell, and the solution bubbles vigorously. When the cell is fully charged (about 2.2 volts), the switch is thrown to the right, and the electric bell rings (drawing about 200 ma). When the cell is being charged, the negative electrode (cathode) attracts positive hydrogen ions. The charged hydrogen ions are neutralized, and hydrogen gas bubbles out of the solution at the cathode. The positive electrode (anode) attracts the negative SO4 ions, which in turn take hydrogen from water molecules to produce more sulfuric acid. The remaining negative oxygen ions unite chemically with the anode to form a layer of reddish-brown lead oxide. When the charged cell is placed across the electric bell, the lead dioxide plate becomes the anode. While the cell is discharging, the lead dioxide on the anode is converted into lead sulphate and water. When both electrodes become covered with lead sulphate, no more current flows in the cell. The process is reversible by recharging the cell.
UCB Index:
D+80+6
PIRA Index:
5E40.60
UCB Taxonomy:
PIRA Taxonomy:
Popularity:
- Log in to post comments