Gay-Lussac's Law: At constant V, Pressure is proportional to Temperature.
Primary tabs
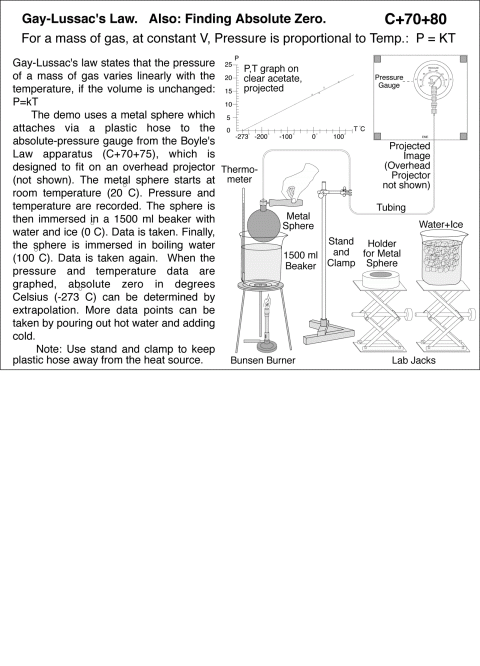
Gay-Lussac's Law. Also: Finding Absolute Zero. For a mass of gas, at constant V, Pressure is proportional to Temp.: P = KT. Gay-Lussac's law states that the pressure of a mass of gas varies linearly with the temperature, if the volume is unchanged: P=kT The demo uses a metal sphere which attaches via a plastic hose to the absolute-pressure gauge from the Boyle's Law apparatus (C+70+75), which is designed to fit on an overhead projector (not shown). The metal sphere starts at room temperature (20 C). Pressure and temperature are recorded. The sphere is then immersed in a 1500 ml beaker with water and ice (0 C). Data is taken. Finally, the sphere is immersed in boiling water (100 C). Data is taken again. When the pressure and temperature data are graphed, absolute zero in degrees Celsius (-273 C) can be determined by extrapolation. More data points can be taken by pouring out hot water and adding cold. Note: Use stand and clamp to keep plastic hose away from the heat source. THERMOMETRY
UCB Index:
C+70+80
PIRA Index:
4E30.10
UCB Taxonomy:
PIRA Taxonomy:
Video:
Popularity:
- Log in to post comments