Charles' Law: At constant P, Volume is proportional to Temperature.
Primary tabs
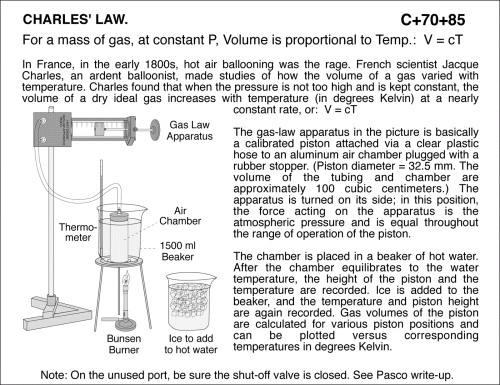
Charles' Law: At constant P, Volume is proportional to Temperature. Apparatus Note: On the unused port, be sure the shut-off valve is closed. See Pasco write-up. In France, in the early 1800s, hot air ballooning was the rage. French scientist Jacque Charles, an ardent balloonist, made studies of how the volume of a gas varied with temperature. Charles found that when the pressure is not too high and is kept constant, the volume of a dry ideal gas increases with temperature (in degrees Kelvin) at a nearly constant rate, or: V = cT The gas-law apparatus in the picture is basically a calibrated piston attached via a clear plastic hose to an aluminum air chamber plugged with a rubber stopper. (Piston diameter = 32.5 mm. The volume of the tubing and chamber are approximately 100 cubic centimeters.) The apparatus is turned on its side; in this position, the force acting on the apparatus is the atmospheric pressure and is equal throughout the range of operation of the piston. The chamber is placed in a beaker of hot water. After the chamber equilibrates to the water temperature, the height of the piston and the temperature are recorded. Ice is added to the beaker, and the temperature and piston height are again recorded. Gas volumes of the piston are calculated for various piston positions and can be plotted versus corresponding temperatures in degrees Kelvin. THERMOMETRY
UCB Index:
C+70+85
UCB Taxonomy:
Video:
Popularity:
- Log in to post comments