You are here
Main menu
- Home
- Request History
- Links to Applets
- Demonstrations By UCB Index
- Mechanics (A)
- Acceleration (A+0)
- Conservation of Energy (A+5)
- Frames of Reference (A+10)
- Friction (A+12)
- Forces (A+14)
- Gravitation (A+15)
- Linear Inertia (A+20)
- Rotational Inertia (A+25)
- Angular Momentum (A+30)
- Linear Momentum (A+35)
- Motion in One Dimension (A+37)
- Physical Measurements (A+45)
- Projectiles (A+50)
- Rotational Dynamics (A+55)
- Statics and Mechanical Equilibrium (A+60)
- Torque (A+65)
- Vectors (A+70)
- Mechanical Advantage (A+80)
- Waves (B)
- Chaotic Oscillations (B+5)
- Simple Harmonic Motion (B+10)
- Coupled Harmonic Oscillators (B+15)
- Forced Oscillations/Resonance (B+20)
- Travelling Waves (B+25)
- Superposition : Fourier Principles/Complex Waves (B+30)
- Interference (B+35)
- Sound Spectrum/Sources (B+45)
- Standing Waves/Resonance (B+50)
- Vibrational Modes (B+55)
- Speed of Sound (B+60)
- Doppler Shift (B+65)
- Shock Waves (B+67)
- Music and the Ear (B+70)
- Properties of Heat and Matter (C)
- Weather and the Atmosphere (C+0)
- Calorimetry (C+5)
- Carnot Cycle (C+10)
- Conduction (C+15)
- Convection (C+20)
- Engines and Pumps (C+22)
- Fluid Dynamics (C+25)
- Surface Tension and Capillary Action (C+27)
- Fluid Statics (C+30)
- Heat and Work (C+35)
- Heat Capacity (C+40)
- Heat of Fusion (C+45)
- Irreversibility and Fluctuations (C+50)
- Kinetic Theory and Gas Models (C+55)
- Liquification of a Gas (C+60)
- Molecular Models and Crystal Structure (C+62)
- Radiation (C+65)
- Temperature and Expansion (C+70)
- Thermometry (C+75)
- Triple Point (C+80)
- Mechanical Properties of Materials (C+90)
- Electricity and Magnetism (D)
- Capacitance (D+0)
- Electromagnetic Oscillations (D+5)
- Electrostatics (D+10)
- Faraday's Law (D+15)
- Inductance (D+20)
- LCR Phase Relationships (D+25)
- Magnetic Fields (D+30)
- Magnetic Properties (D+35)
- Meters (D+40)
- Motors (D+45)
- Oscilloscopes (D+50)
- Resistance (D+55)
- Solid State and Semiconductors (D+60)
- Thermionic Emission (D+65)
- Thermoelectricity (D+70)
- Transformers (D+75)
- Voltaic Cells (D+80)
- Electrolysis (D+85)
- Optics (E)
- Modern and Contemporary Physics (F)
- Cathode and Canal Rays (F+0)
- X-Rays (F+5)
- Electron Diffraction (F+10)
- Photoelectric Effect (F+15)
- Atomic Structure (F+20)
- Photons (F+18)
- Quantum Mechanical Barrier Penetration (F+30)
- Scattering (F+25)
- Elementary Particles (F+35)
- Cloud Chambers (F+45)
- Range of Alpha Particles (F+50)
- Franck-Hertz Experiment (F+55)
- Radioactivity (F+65)
- Accelerators (F+70)
- Fission and Fusion (F+80)
- Superconductivity (F+85)
- Zeeman Effect (F+90)
- Laser (F+95)
- Fuel Cells (F+100)
- Astronomy and Perception (G)
- Mechanics (A)
- Demonstrations by PIRA index
- 1 Mechanics
- 1A Measurement
- 1C Motion In One Dimension
- 1D Motion In Two Dimensions
- 1E Relative Motion
- 1F Newton's First Law
- 1G Newton's Second Law
- 1H Newton's Third Law
- 1J Statics Of Rigid Bodies
- 1K Applications Of Newton's Laws
- 1L Gravity
- 1M Work And Energy
- 1N Linear Momentum And Collisions
- 1Q Rotational Dynamics
- 1R Properties Of Matter
- 3 Oscillations and Waves
- 3A Oscillations
- 3B Wave Motion
- 3B10 Transverse Pulses and Waves
- 3B20 Longitudinal Pulses and Waves
- 3B22 Standing Waves
- 3B25 Impedence and Dispersion
- 3B27 Compound Waves
- 3B30 Wave Properties of Sound
- 3B33 Phase and Group Velocity
- 3B35 Reflection & Refraction (Sound)
- 3B39 Transfer of Energy in Waves
- 3B40 Doppler Effect
- 3B45 Shock Waves
- 3B50 Interference and Diffraction
- 3B55 Interference and Diffraction Of Sound
- 3B60 Beats
- 3B70 Coupled Resonators
- 3C Acoustics
- 3D Instruments
- 3E Sound Reproduction
- 4 Thermodynamics
- 5 Electricity and Magnetism
- 6 Optics
- 7 Modern Physics
- 8 Astronomy
- 8A Planetary Astronomy
- 8A05 Historical Astronomy
- 8A10 Solar System Mechanics
- 8A20 Earth Moon Mechanics
- 8A30 Views From Earth
- 8A35 Views From Earth 2
- 8A40 Planetary Properties: Globes, Hemispheres, & Maps
- 8A50 Planetary Properties 2: The Planets
- 8A60 Planetary Properties 3: Planetoids, Minor Objects
- 8A70 Planetary Properties 4: Planetary Characteristics
- 8A80 Planetary Properties 5: Comets And The Search For Life
- 8B Stellar Astronomy
- 8C Cosmology
- 8D Miscellaneous
- 8E Astronomy Teaching Techniques
- 8A Planetary Astronomy
- 9 Equipment
- 9A Support Systems / Facility
- 9A10 Blackboard Tools
- 9A20 Audio
- 9A30 Slide Projectors
- 9A34 Film Projectors
- 9A36 Overhead Projectors
- 9A38 Video & Computer Projection
- 9A40 Photography
- 9A50 X-Y, Chart Recorders
- 9A60 Buildings
- 9A65 Museums
- 9A70 Resource Books
- 9A73 Unclassified Demonstrations
- 9A75 Philosophy
- 9A80 Films
- 9A85 Computer Programs
- 9B Electronic
- 9B10 Timers
- 9B15 Position and Velocity Detectors
- 9B17 Sources of Sound
- 9B18 Sound Detectors
- 9B20 Circuits/Components/Instruments
- 9B30 Function Generators
- 9B37 Oscilloscopes
- 9B40 Advanced Instruments
- 9B50 Power Supplies
- 9B60 Light Sources
- 9B61 Light Paths Made Visible
- 9B62 Lasers
- 9B65 Microwave Apparatus
- 9B90 Computer Interface
- 9C Mechanical
- 9A Support Systems / Facility
- 2 Fluid Mechanics
- 1 Mechanics
User login
Boyle's Law: At constant T, Pressure times Volume is a constant.
Primary tabs
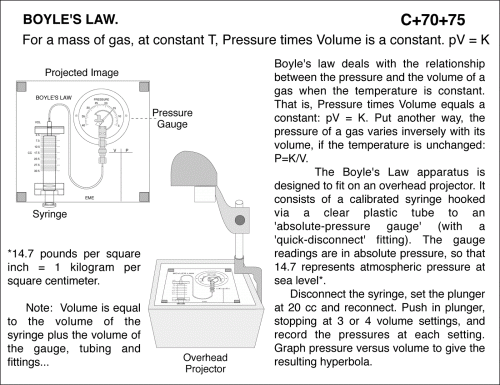
For a mass of gas, at constant T, Pressure times Volume is a constant. pV = K. Boyle's law deals with the relationship between the pressure and the volume of a gas when the temperature is constant. That is, Pressure times Volume equals a constant: pV = K. Put another way, the pressure of a gas varies inversely with its volume, if the temperature is unchanged: P=K/V. The Boyle's Law apparatus is designed to fit on an overhead projector. It consists of a calibrated syringe hooked via a clear plastic tube to an 'absolute-pressure gauge' (with a 'quick-disconnect' fitting). The gauge readings are in absolute pressure, so that 14.7 represents atmospheric pressure at sea level*. Disconnect the syringe, set the plunger at 20 cc and reconnect. Push in plunger, stopping at 3 or 4 volume settings, and record the pressures at each setting. Graph pressure versus volume to give the resulting hyperbola. *14.7 pounds per square inch = 1 kilogram per square centimeter. Note: Volume is equal to the volume of the syringe plus the volume of the gauge, tubing and fittings
UCB Index:
C+70+75
PIRA Index:
4E20.10
UCB Taxonomy:
PIRA Taxonomy:
Video:
Popularity:
Previous:
- Log in to post comments