You are here
Main menu
- Home
- Request History
- Links to Applets
- Demonstrations By UCB Index
- Mechanics (A)
- Acceleration (A+0)
- Conservation of Energy (A+5)
- Frames of Reference (A+10)
- Friction (A+12)
- Forces (A+14)
- Gravitation (A+15)
- Linear Inertia (A+20)
- Rotational Inertia (A+25)
- Angular Momentum (A+30)
- Linear Momentum (A+35)
- Motion in One Dimension (A+37)
- Physical Measurements (A+45)
- Projectiles (A+50)
- Rotational Dynamics (A+55)
- Statics and Mechanical Equilibrium (A+60)
- Torque (A+65)
- Vectors (A+70)
- Mechanical Advantage (A+80)
- Waves (B)
- Chaotic Oscillations (B+5)
- Simple Harmonic Motion (B+10)
- Coupled Harmonic Oscillators (B+15)
- Forced Oscillations/Resonance (B+20)
- Travelling Waves (B+25)
- Superposition : Fourier Principles/Complex Waves (B+30)
- Interference (B+35)
- Sound Spectrum/Sources (B+45)
- Standing Waves/Resonance (B+50)
- Vibrational Modes (B+55)
- Speed of Sound (B+60)
- Doppler Shift (B+65)
- Shock Waves (B+67)
- Music and the Ear (B+70)
- Properties of Heat and Matter (C)
- Weather and the Atmosphere (C+0)
- Calorimetry (C+5)
- Carnot Cycle (C+10)
- Conduction (C+15)
- Convection (C+20)
- Engines and Pumps (C+22)
- Fluid Dynamics (C+25)
- Surface Tension and Capillary Action (C+27)
- Fluid Statics (C+30)
- Heat and Work (C+35)
- Heat Capacity (C+40)
- Heat of Fusion (C+45)
- Irreversibility and Fluctuations (C+50)
- Kinetic Theory and Gas Models (C+55)
- Liquification of a Gas (C+60)
- Molecular Models and Crystal Structure (C+62)
- Radiation (C+65)
- Temperature and Expansion (C+70)
- Thermometry (C+75)
- Triple Point (C+80)
- Mechanical Properties of Materials (C+90)
- Electricity and Magnetism (D)
- Capacitance (D+0)
- Electromagnetic Oscillations (D+5)
- Electrostatics (D+10)
- Faraday's Law (D+15)
- Inductance (D+20)
- LCR Phase Relationships (D+25)
- Magnetic Fields (D+30)
- Magnetic Properties (D+35)
- Meters (D+40)
- Motors (D+45)
- Oscilloscopes (D+50)
- Resistance (D+55)
- Solid State and Semiconductors (D+60)
- Thermionic Emission (D+65)
- Thermoelectricity (D+70)
- Transformers (D+75)
- Voltaic Cells (D+80)
- Electrolysis (D+85)
- Optics (E)
- Modern and Contemporary Physics (F)
- Cathode and Canal Rays (F+0)
- X-Rays (F+5)
- Electron Diffraction (F+10)
- Photoelectric Effect (F+15)
- Atomic Structure (F+20)
- Photons (F+18)
- Quantum Mechanical Barrier Penetration (F+30)
- Scattering (F+25)
- Elementary Particles (F+35)
- Cloud Chambers (F+45)
- Range of Alpha Particles (F+50)
- Franck-Hertz Experiment (F+55)
- Radioactivity (F+65)
- Accelerators (F+70)
- Fission and Fusion (F+80)
- Superconductivity (F+85)
- Zeeman Effect (F+90)
- Laser (F+95)
- Fuel Cells (F+100)
- Astronomy and Perception (G)
- Mechanics (A)
- Demonstrations by PIRA index
- 1 Mechanics
- 1A Measurement
- 1C Motion In One Dimension
- 1D Motion In Two Dimensions
- 1E Relative Motion
- 1F Newton's First Law
- 1G Newton's Second Law
- 1H Newton's Third Law
- 1J Statics Of Rigid Bodies
- 1K Applications Of Newton's Laws
- 1L Gravity
- 1M Work And Energy
- 1N Linear Momentum And Collisions
- 1Q Rotational Dynamics
- 1R Properties Of Matter
- 3 Oscillations and Waves
- 3A Oscillations
- 3B Wave Motion
- 3B10 Transverse Pulses and Waves
- 3B20 Longitudinal Pulses and Waves
- 3B22 Standing Waves
- 3B25 Impedence and Dispersion
- 3B27 Compound Waves
- 3B30 Wave Properties of Sound
- 3B33 Phase and Group Velocity
- 3B35 Reflection & Refraction (Sound)
- 3B39 Transfer of Energy in Waves
- 3B40 Doppler Effect
- 3B45 Shock Waves
- 3B50 Interference and Diffraction
- 3B55 Interference and Diffraction Of Sound
- 3B60 Beats
- 3B70 Coupled Resonators
- 3C Acoustics
- 3D Instruments
- 3E Sound Reproduction
- 4 Thermodynamics
- 5 Electricity and Magnetism
- 6 Optics
- 7 Modern Physics
- 8 Astronomy
- 8A Planetary Astronomy
- 8A05 Historical Astronomy
- 8A10 Solar System Mechanics
- 8A20 Earth Moon Mechanics
- 8A30 Views From Earth
- 8A35 Views From Earth 2
- 8A40 Planetary Properties: Globes, Hemispheres, & Maps
- 8A50 Planetary Properties 2: The Planets
- 8A60 Planetary Properties 3: Planetoids, Minor Objects
- 8A70 Planetary Properties 4: Planetary Characteristics
- 8A80 Planetary Properties 5: Comets And The Search For Life
- 8B Stellar Astronomy
- 8C Cosmology
- 8D Miscellaneous
- 8E Astronomy Teaching Techniques
- 8A Planetary Astronomy
- 9 Equipment
- 9A Support Systems / Facility
- 9A10 Blackboard Tools
- 9A20 Audio
- 9A30 Slide Projectors
- 9A34 Film Projectors
- 9A36 Overhead Projectors
- 9A38 Video & Computer Projection
- 9A40 Photography
- 9A50 X-Y, Chart Recorders
- 9A60 Buildings
- 9A65 Museums
- 9A70 Resource Books
- 9A73 Unclassified Demonstrations
- 9A75 Philosophy
- 9A80 Films
- 9A85 Computer Programs
- 9B Electronic
- 9B10 Timers
- 9B15 Position and Velocity Detectors
- 9B17 Sources of Sound
- 9B18 Sound Detectors
- 9B20 Circuits/Components/Instruments
- 9B30 Function Generators
- 9B37 Oscilloscopes
- 9B40 Advanced Instruments
- 9B50 Power Supplies
- 9B60 Light Sources
- 9B61 Light Paths Made Visible
- 9B62 Lasers
- 9B65 Microwave Apparatus
- 9B90 Computer Interface
- 9C Mechanical
- 9A Support Systems / Facility
- 2 Fluid Mechanics
- 1 Mechanics
User login
Mechanical models of the hydrogen atom.
Primary tabs
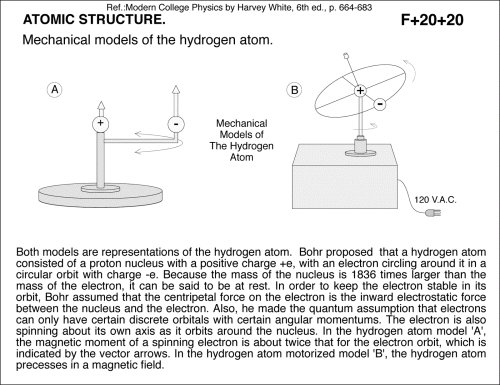
Mechanical models of the hydrogen atom. Both models are representations of the hydrogen atom. Bohr proposed that a hydrogen atom consisted of a proton nucleus with a positive charge +e, with an electron circling around it in a circular orbit with charge -e. Because the mass of the nucleus is 1836 times larger than the mass of the electron, it can be said to be at rest. In order to keep the electron stable in its orbit, Bohr assumed that the centripetal force on the electron is the inward electrostatic force between the nucleus and the electron. Also, he made the quantum assumption that electrons can only have certain discrete orbitals with certain angular momentums. The electron is also spinning about its own axis as it orbits around the nucleus. In the hydrogen atom model 'A', the magnetic moment of a spinning electron is about twice that for the electron orbit, which is indicated by the vector arrows. In the hydrogen atom motorized model 'B', the hydrogen atom precesses in a magnetic field. Ref.:Modern College Physics by Harvey White, 6th ed., p. 664-683
UCB Index:
F+20+20
PIRA Index:
7B50.10
UCB Taxonomy:
PIRA Taxonomy:
Popularity:
- Log in to post comments