UV light hits charged zinc plate and discharges electroscope.
Primary tabs
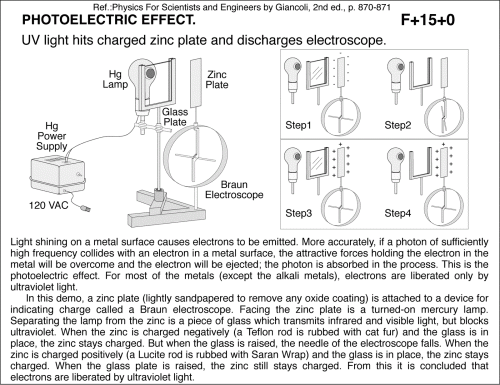
UV light hits charged zinc plate and discharges electroscope. Light shining on a metal surface causes electrons to be emitted. More accurately, if a photon of sufficiently high frequency collides with an electron in a metal surface, the attractive forces holding the electron in the metal will be overcome and the electron will be ejected; the photon is absorbed in the process. This is the photoelectric effect. For most of the metals (except the alkali metals), electrons are liberated only by ultraviolet light. In this demo, a zinc plate (lightly sandpapered to remove any oxide coating) is attached to a device for indicating charge called a Braun electroscope. Facing the zinc plate is a turned-on mercury lamp. Separating the lamp from the zinc is a piece of glass which transmits infrared and visible light, but blocks ultraviolet. When the zinc is charged negatively (a Teflon rod is rubbed with cat fur) and the glass is in place, the zinc stays charged. But when the glass is raised, the needle of the electroscope falls. When the zinc is charged positively (a Lucite rod is rubbed with Saran Wrap) and the glass is in place, the zinc stays charged. When the glass plate is raised, the zinc still stays charged. From this it is concluded that electrons are liberated by ultraviolet light. Ref.:Physics For Scientists and Engineers by Giancoli, 2nd ed., p. 870-871
UCB Index:
F+15+0
PIRA Index:
7A10.10
UCB Taxonomy:
PIRA Taxonomy:
Video:
Popularity:
- Log in to post comments