Copper nail and iron nail in a lemon using a multimeter.
Primary tabs
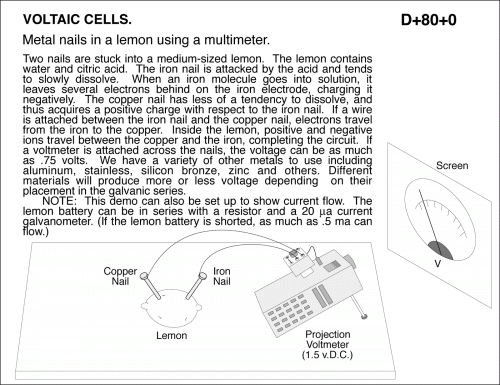
Metal nails in a lemon using a multimeter. Two nails are stuck into a medium-sized lemon. The lemon contains water and citric acid. The iron nail is attacked by the acid and tends to slowly dissolve. When an iron molecule goes into solution, it leaves several electrons behind on the iron electrode, charging it negatively. The copper nail has less of a tendency to dissolve, and thus acquires a positive charge with respect to the iron nail. If a wire is attached between the iron nail and the copper nail, electrons travel from the iron to the copper. Inside the lemon, positive and negative ions travel between the copper and the iron, completing the circuit. If a voltmeter is attached across the nails, the voltage can be as much as .75 volts. We have a variety of other metals to use including aluminum, stainless, silicon bronze, zinc and others. Different materials will produce more or less voltage depending on their placement in the galvanic series. NOTE: This demo can also be set up to show current flow. The lemon battery can be in series with a resistor and a 20 _a current galvanometer. (If the lemon battery is shorted, as much as .5 ma can flow.)
UCB Index:
D+80+0
PIRA Index:
5D40.25
Demo Picture:
UCB Taxonomy:
PIRA Taxonomy:
Popularity:
Previous:
- Log in to post comments