Diffusion of Ammonia and HCl.
Primary tabs
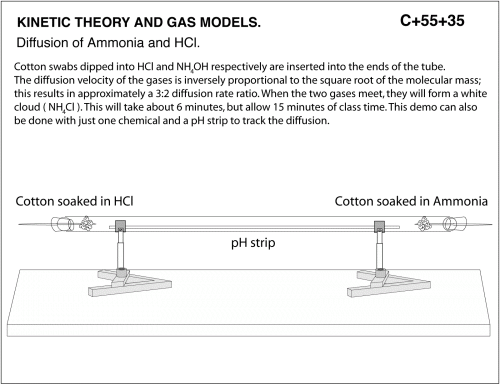
Diffusion of Ammonia and HCl. Cotton swabs dipped into HCl and NH40H respectively are inserted into the ends of the tube. The diffusion velocity of the gases is inversely proportional to the square root of the molecular mass; this results in approximately 3:2 diffusion rate ratio. When the two gases meet, tey will form a white cloud (NH4Cl). this will take about 6 minutes, but allow 15 minutes of class time. This demo can also be done with just one chemical and a pH strip to track the diffusion.
UCB Index:
C+55+35
Demo Picture:

UCB Taxonomy:
Popularity:
- Log in to post comments